According to CDC (Guidelines for Health Professionals) the recommended methods for the confirmation of suspected cases of novel Influenza A H1N1 are real time PCR and viral culture followed by sequencing.
For those laboratories performing viral culture, shell-vial technique offers the advantages of easy use, reading in 24 hours and minimum equipment required.
From today Vircell offers the following products:
|
Ref. |
Name |
No. of tests |
Description |
Content |
|---|---|---|---|---|
|
ACINFA |
Influenza A |
40 tests |
Staining kit for Influenza A using Immunofluorescence technique. |
1 ml of specific monoclonal antibody (FITC- labelled) and positive and negative controls |
|
KINFA |
Inoculation and staining kit for Influenza A |
24 tests |
Inoculation and staining kit for Influenza A using the Immunofluorescence technique. |
Shell-vials of MDCK cell line, 1 ml of specific monoclonal antibody (FITC- labelled), controls, antibiotic mixture, and inoculation medium |
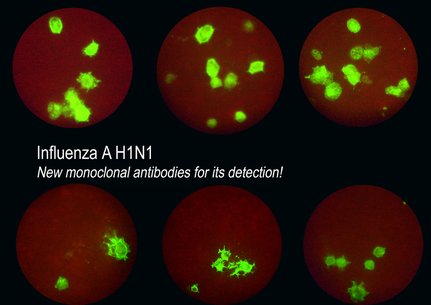
These new products can be used to detect the presence of the new virus Influenza A H1N1 (and seasonal flu) and they allow to quantify viral loads with an immunofluorescence assay (see picture attached). The prices are the same as the ACINF and KINF references.
Should you need further information of FOC samples, please do not hesitate to contact us.
We thank you in advance for your cooperation by informing your existing customers about these products and reaching new potential customers.